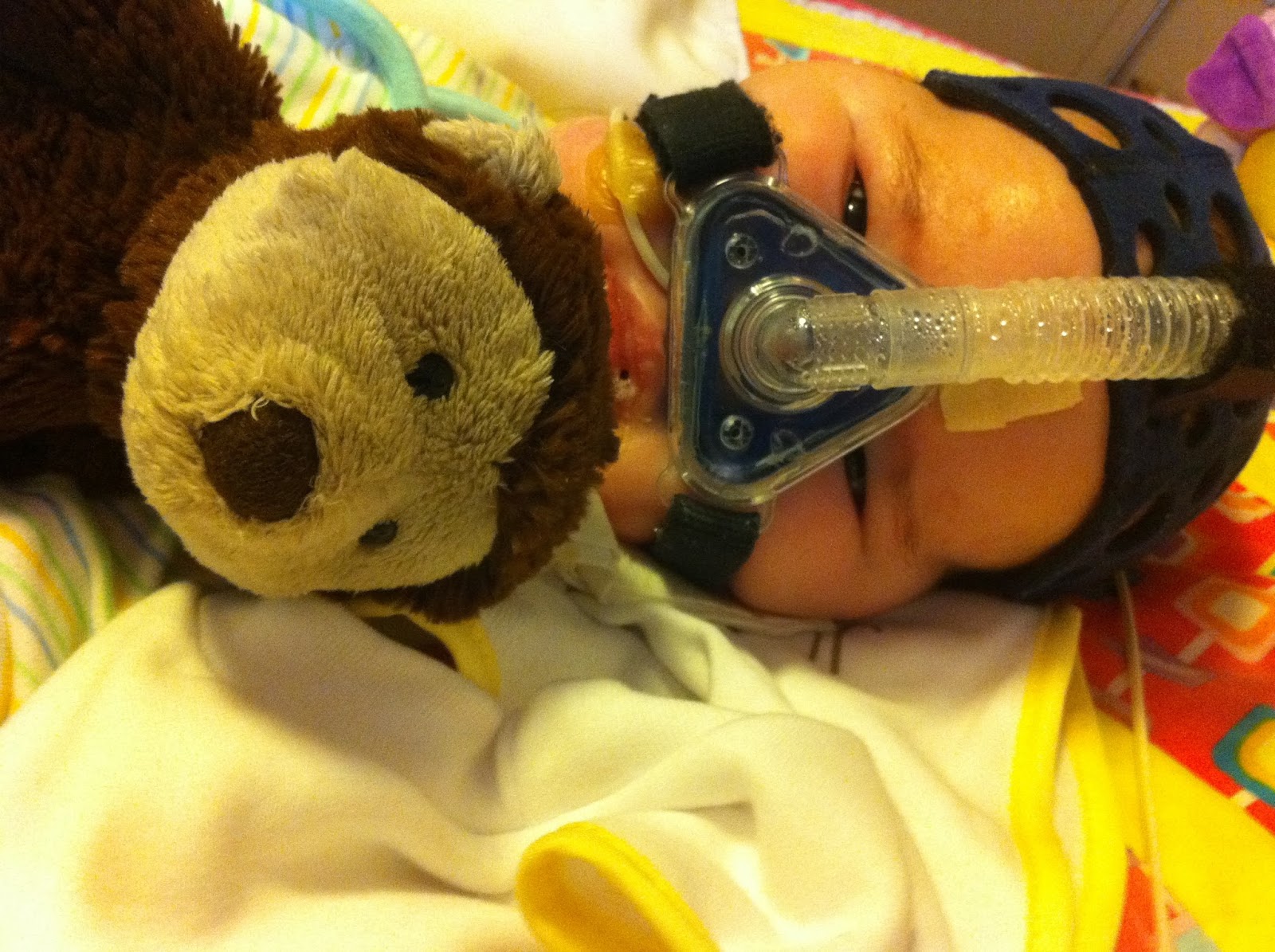
It's been awhile
Three things happened during the last week that have occasioned this blog.
Firstly we were in Seville for a break and had gone to Estella's fountain. We bought her her usual present and put it in the water. It was a water pistol because I imagine she's rather cheeky by now and I had a trial shooting water at her Mummy to amuse her. As we left Maria asked why I had not taken photos this time for Twitter and I said it did not feel right. She was happy and said that it was a sign that she was becoming our daughter again and not a symbol for a campaign.
Secondly we heard from the organisation that are doing the consultation into screening for SMA that we had been fighting for. They apologised for overlooking us in the consultation process and suggested that they would extend the consultation by two months. All I could think was that would be another two month delay until the outcomes were realised and I also decided that i think I have said all I want to say.
Thirdly - It is SMA awareness month. I found this out from my own twitter account on August 7th. It was then that I realised I was no longer paying the attention that was needed to an account with 44,000 followers. I simply couldn't find the 2-3 hours a day I used to find.
All of this sounds as though I am ending the SMASHSMA campaign.
I'm not.
I intend to raise funds when I can. I intend to spread awareness, I will be there instantly if any parents ever needs help or support, I intend to carry on raising the matter with the government but I feel sure you can see I have not been around as much lately and wanted to explain that the moments we are sharing with Cristina are absolute heaven and I would be wrong to use my energy fighting SMA each and every day. I need to be a lot more focused. Cristina is bringing so much joy and yet it is tinged with sadness. I watch her now starting to crawl and laughing and holding her own spoon and I realise more than ever how poorly Estella was. I see what was going on in her head and it makes me cry. It strips me down to the same raw feeling that we had when Estella died.
I promised Esetlla that I would do all I could to rid the world of this terrible disease and I'm not giving up on that but I am saying that there will not be 50 tweets a day anymore.
It's more than a matter of time though
I have called this blog Heroes - because of the people we have met along the way. The individuals and organisations who have done so much to spread the SMA awareness story. I'm not going to start listing because I will miss somebody out and I would hate to do that. We have met some exceptional people and had extraordinary support. We have had one organisation ( Morrison Facility Services) that have spent a fortune - we have had individuals who have tweeted all night every night for month after month, we have had some medical support while Estella was alive from people who loved her and became friends.
There have been low points. The cynical politician who tried to hijack the story, the celebrity who disappeared and some amazing behaviour from the charities that are supposed to be fighting this disease. It would be churlish to go into detail but if I have had one single life-changing experience it has been with the incompetence demonstrated by a major SMA support charity. I genuinely believe that it is not from lack of money that SMA is not cured yet but from a lack of direction , intelligence, innovation and desire from some of the most unprofessional morons it has ever been my displeasure to speak with. They know who they are.
Time wasters - money wasters - life wasters. Shameless
Let's not end this blog with that bitterness that i feel towards some of the organisations.
It's called HEROES for a reason.
Let's end with an abiding thought of the sweet, dear little girl that this is all about. Our Estella was an exceptional person by any standard. She lived a short and meaningful life and she made a difference to so many people. She inspired, she entertained and she shone like an absolute burning star.
I miss her every day. I think of her constantly and I would give my heart and soul for another ten minutes with her. She never ran, she never sang, she never stood and played with a ball - but she is in my heart and my mind and my life. She was born with the cruellest , most wicked , disease it is possible to imagine and yet she smiled and brought so much happiness into our lives.
Estella means star.
There are so many stars in the sky. There are children everyday who are dying from this disease. I will continue to do what I can - but - it's not my life anymore, it's not my reason for being anymore - remembering Estella and loving my time with Cristina and Maria is my life now. They're my three girls. They're my life - and SMA is a dirty , nasty - naughty little disease. If we can make something of this life and find happiness then Estella beat SMA. If we can be happy then that little heroic girl did something that I could not.
She smashed SMA
Thankyou for listening
And for all your kindness to my family
May you always be happy.
SMASH SMA
A blog about the SMASHSMA campaign to raise awareness of Spinal Muscular Atrophy the number one genetic killer of children under 2. In memory of Estella who died in November 2011
Thursday, August 8, 2013
Monday, July 8, 2013
SMA - THE CONSULTATION
Had the following through from Edd Hair
Assistant Private Secretary, Minister of State for Care and Support
This is the follow through to the meeting we had with The Minister and the Deputy PM last year.
I hope that some of you find time to give response to teh consultation.
Tom
Further to my email below, the minister asked me to keep a watching brief on policy relating to spinal muscular atrophy, and to write back to you when I had something helpful to pass on.
First of all, I wanted to make sure you were aware that the UK National Screening Committee’s (UKNSC’s) consultation on screening for SMA was now underway. The review of screening policy for SMA is taking place from May 2013 to May 2014, and the UKNSC are seeking views by email until 5 August. Further details are available on their website, and I would encourage you and your supporters to write in with your views:
I know one of the other areas of focus for your campaign was funding for research. In particular, you mentioned the work of Professor Gillingwater's research team at the University of Edinburgh. The overall focus of their research is on cellular and molecular mechanisms that regulate the form and function of the nervous system in health and disease. The UK Research Councils are the appropriate public funders of this type of research, and the research team's webpages acknowledge funding support from the Biotechnology and Biological Sciences Research Council.
The Department of Health funds applied health research in England through the National Institute for Health Research (NIHR). The NIHR biomedical research centre at Great Ormond Street Hospital and the Institute of Child Health has carried out research on spinal muscular atrophy. Applied health research in Scotland is funded by the Chief Scientific Office.
I’m sure you / Professor Gillingwater will have already pursued a number of funding opportunities, but in case not, here are further details of the funding opportunities provided by relevant organisations:
These organisations are independent and do not ring-fence funding for particular projects, however all organisations consider applications in open competition, with awards being made on the basis of the scientific quality of the proposals made. Decisions are taken independently, according to the long-established ‘Haldane principle’. If Professor Gillingwater has not already done so, I would certainly encourage applications to these various funding sources. I’m sure the minister would want me to pass on his apologies that he can’t be more directly helpful with influencing funding bodies – which I know was the central point of your email below - but I’m sure you will appreciate the importance funding decisions being made on merit and without the interference of ministers.
You might also be interested to know that the National Institute for Health and Care Excellence (NICE) has been asked to develop a clinical guideline on neurological problems. The guideline will provide the underpinning source of evidence for a quality standard on the same topic. (Both documents effectively set out what good care looks like, and are used by CCGs to inform the services they commission).
The exact scope of the guidance, including which conditions will and will not be covered, will be determined by NICE through consultation with stakeholders, but you may wish to keep abreast of work on this topic as it develops. Once NICE initiates work on this guidance, further information will be available on NICE's website at http://www.nice.org.uk/guidance/CG/InDevelopment. You can see ‘neurological problems’ on the list of guidelines referred on this page: http://www.nice.org.uk/guidance/cg/indevelopment/GuidelineReferralsUnderpinStandards.jsp and may want to write to NICE to seek further confirmation for exactly when they intend to develop this guidance further, and to ask that you are engaged / can become involved at that stage.
Finally, you may be aware that NHS England took on their formal responsibilities as of 1st April this year. This means that, in effect, NHS England is responsible for determining clinical policy (including policy on the diagnosis and treatment of spinal muscular atrophy). The government sets out a high level ‘Mandate’ to NHS England which sets out strategic priorities on a general level (including ‘domain two’ which focuses on long term conditions); and it is for NHS England to determine how to deliver against the objectives set out in the Mandate.
You can view the Mandate here: http://mandate.dh.gov.uk/
You might also wish to write to NHS England for more information on their intentions around clinical policy in this area. The relevant contact would probably be David Bateman, who is the National Clinical Director for acute and chronic neurological disabilities.
I hope the above is of interest, and apologise for not having been in touch sooner.
Yours faithfully,
Edd
Edmund Hair
Assistant Private Secretary, Minister of State for Care and Support
Department of Health, 79 Whitehall, SW1A 2NS
|
Saturday, June 8, 2013
LET LIAM LIVE - AN UPDATE
We have had two replies from Liam's Dad both of which show what a humble and intelligent person he is. In the first one he is keen to say that they do not wish to attack ISIS pharmaceuticals but would rather try persuasion. We have made some suggestions.
In the second he replied as follows
Hi there,
In the second he replied as follows
Hi there,
Thank you for your support, your encouragement given us a great ideas, I think, we now have a plan to make something big.
Obviously we will work quickly, because each day count in the SMA. In waiting if I understand we need to put into our communications the #smashsma ,
#letliamlive and the link to your blog?
Give us a few days and the Canadians will be to the appointment:-)
#letliamlive and the link to your blog?
Give us a few days and the Canadians will be to the appointment:-)
I replied with
Excellent. You can use any of our information. You can use #smashsma #letliamlive or our blog if it helps. I will write to or speak to anybody you suggest. I will do every single thing I possibly can to help you. It is essential that your son is given a chance. You have my 100% commitment to your cause. I have contacted a friend in the American Embassy. I think you are right it is proper to be polite with ISIS but they will listen better when you and that lovely boy are on television. Take care and - I hope this sounds proper - despite all of these things enjoy every moment with your little fella because these are special days that will not come again. You know that I am sure but I wanted to say it.
Tom
Friday, June 7, 2013
CAN WE SAVE THIS LIFE ?
THERE IS AN 8 MONTH OLD BOY AND HE NEEDS YOU- PLEASE READ
You may have seen an article about a little boy called Liam who is 8 months old and has SMA. The trials that we have been fighting for are STARTING in Canada and SMA type 1 babies are being given a chance to live - apart from Liam because he is 17 days too old to qualify for the trial. You may have heard this story - well today we received a direct appeal from Liam's parents asking if there is anything we can do.
Please can I ask you to make a fuss about this - let's make a noise all over TWITTER. The hash tag will be #letliamlive LET LIAM LIVE. Even if he is outside the age for the trial he should be allowed the drug and the results should then not be included in the study. There are not thousand's of these babies there is this one little lad and they are going to let him have no chance of regeneration because he is 17 days too old.
This is barbaric.
Here's the story
LET LIAM LIVE
MONTREAL - An infant who's slowly dying of a rare spinal defect has been denied an experimental medical treatment because he's 17 days too old.
The cutoff set by the U.S. pharmaceutical firm ISIS has outraged his parents, Yan Defosses and Emanuelle Desbiens.
"It's cruel and completely inhuman," Defosses told QMI Agency.
Eight-month-old Liam suffers from spinal muscular atrophy, a genetic disease that attacks the
His parents realized something was wrong soon after his birth. He was losing muscle tone instead of gaining it.
When he was four months old, doctors gave his parents the diagnosis and said the baby only had two years to live.
He's already showing
But the family's hopes were kindled when they learned that California-based ISIS has conducted clinical trials on the drug SMNRx.
"We are in contact with families in the United States, and it has completely changed the lives of their children," said Defosses. "They've survived under more than acceptable conditions."
Earlier this spring, the company announced a second phase of clinical trials, this time in babies. But the children had to be seven months old or less before May 1. Liam was seven months and 17 days old.
Liam's doctors said they could do nothing because the trials aren't being conducted in Canada.
Saint-Justine Hospital and Health Canada were prepared to approve Liam's treatment but ISIS refused.
"It shocked us and destroyed us," said Defosses.
Below is the heart-breaking plea from his Dad
Please tweet about this - let's start the ball rolling
Please tweet ISIS - Please allow Liam to be included in your SMA study. Please let hum live #letliamlive
-------- Original Message --------
Subject: Message via votre profil Google : We need your help
From: Yan Défossés <yan@groupeinfiny.com>
To: estellameansstar@gmail.com
CC:
Greeting to you,, my name is Yan I'm the father of a child who are diagnosed with sma type 1. I write to you from Montréal Canada, like you probably know Isis pharmaceutical begin a clinical trial on infant with her compound the SMNRX. Our son Liam meet all the criterias for this study, unfortunately the canadian centre is not yet rolling and my son just turn 8 months. So he is now too hold for the study.
Because we know how it's important to restore as soon as possible the smn protein level, that few weeks, can make all the difference to restore motor function of our child, we continued our research, to avoid delaying access to the experimental treatment. After describing the context and give all the information we have about Isis and SMNRX, to Health Canada, equivalent of the FDA in U.S. we obtain a special authorization granted by Health Canada allowing Isis to provide the drug out off protocol and releasing any liability. With that way, Isis can receive more data and is not obliged to diffuse or account the result in the actual study.
Obviously, there is no obligation for Isis, to accept. And there is the point, she refuse:-( I understand you make some pressure last year to stress Pfizer to invest in the SMA and it seems that it work with her engagement in the RG3039 and Repligen. Could you give us advice to encourage them to change their minds quickly, before the therapeutic window of our little boy has passed? Thank in advance The Desbiens-Défossés family tel: 1.514.583.1247 Thank you so much again for all you do for the SMA and sorry for the strange writing we usually speak french at home Yan
I have replied
Yan
I was devastated to read your story. Our little girl died aged 8 months from the same disease and we have been working since then to get drug trials and now we are told that ISIS say no to your case. The argument you put forward is fair and reasonable and should appeal to rational and caring hearts,
BUT
What you need now is to make a noise
1) We have asked 44,000 twitter followers to tweet their protest at ISIS and we are using the message LET LIAM LIVE #letliamlive
2) We will do the same of FACEBOOK
3) Messages are being left for you on our blog at www.smashsma.blogspot.com
BUT
You are in Canada. Get the local TV station. Get the local newspaper. Get the local radio. Go and sit in the lounge at the TV station until they will hear you and put you on TV. this is a heart-breaking story and they will give you publicity - publicity enough to SHAME ISIS into acting. You need to tell then that thousands of people in the UK are backing you and you need the people of Canada. Liam needs the Repligen and he needs it now. If the facts are not included in the overall trial so what it is a moral and kind act - it is a human act to give the boy the help he needs.
If ISIS will not listen then get your friends and your family to sit in their reception until the TV cameras turn up. You are fighting for the quality of life of your treasure and you must do everything you can. It is not enough for them to use lame arguments about access to the drug. there are not hundreds of babies with the condition so the argument that they have to draw the line somewhere is NOT ACCEPTABLE. I will do anything at all to help from this end. Do you have a TWITTER account ? Please let me know. We are with you - we are praying that sanity sees its way through. Please let us know what happens. please send more photos of Liam to help me spread the word at this end. I lost my little girl and we swore to her that we would SMASHSMA - well she would love it if this became a very concrete example of her legacy. the people here are amazing and they will be writing to you and giving their hearts.
Be strong. Fight
Emmanuelle Desbiens · Polyvalente de Lévis
Emmanuelle Desbiens · Polyvalente de Lévis
We are the Liam's parents and we would like to clarify, that although it may seem cruel, we understand and respect the position of the pharmaceutical to strictly follow the eligibility criteria of their study. Our sadness is that Isis refuses to grant the three experimental doses despite a special authorization granted by Health Canada, the equivalent of compassion access the FDA in the United States. The authorization provided by the Special Access Programme to drug released from all responsibilities this pharmaceutical. Isis could collect additional data without creating noise in the current study. We follow the developpement of this compound and be confident its the good for our child. In addition, if you know this disease (SMA TYPE1) you know that now is the time or never to help.
Friday, May 31, 2013
NICE TO SEE YOU
The care and attention and support that we have had since Estella's death in November 2011 has been well chronicled in these pages. There have been some celebrities, some institutions , but most of all there have been some fantastic people who could not have been closer if they were family. They listened while we cried , they listened while we raged and they listened while we remembered. Exceptional - amazing people. You know perfectly well if it is you that I am talking about. Do not be modest - you know.
We always said that it would be terrific if one day we could meet up with some of our strongest ( and maybe strangest ) supporters. Some came to London with us to lobby Nick Clegg. Others have visited us around the country but there are far more of you that I want to say thankyou to in person.
As you are probably aware we recently decided to hold a SMASHSMA fundraising evening. The theme was unusual - we wanted to reproduce a 1950s Beat Generation 'Happening' - complete with bearded poets and musicians. Think MADMEN during the first season and you won't go far wrong.
So after lots of organisation we are holding a full weekend of retro 50s and 60s entertainment at the New York Stadium in Rotherham - South Yorkshire. The theme is based around the Beat Generation writer Jack Kerouac who wrote the famous novel - On The Road.
What's that got to do with SMA ? Nothing - and that's the point. The novel, On the Road, and the philosophy of the Beats was to live life in the moment. to celebrate , to love, to dance and to enjoy every moment - and that's what we think of when we think of Estella. a little girl who made the most out of every ounce of life that was given her - and although that wasn't much in terms of time - it was so much in terms of quality and the impact she has left behind.
So
We want YOU to come and meet Maria and myself and Cristina at the event. We want to thank YOU personally for all you have done and the love and support you have shown. Even if you have never heard of Kerouac then I can still promise you a Saturday experience you will never forget. We have a band Heath Common and the Thin Man who will really get you going, We have a play that I wrote with my mate Brian called Beat Surrender and we have an evening of song, poetry, dance and craziness that will be unlike anything you have ever seen as you are transported back in time to 1957 and a retro night to remember. If you come to both days there are panels and discussions and films about the beats on the Sunday.
Every penny of profit ( please God ) is going to Professor Gillingwater's SMASHSMA research fund.
More importantly though we would love to meet you and thankyou. there will be a film about Estella and the chance to help spread the word about SMA with us. Please come and say hello.
HOW YOU CAN HELP
1) Go and follow the show on TWITTER now @kerouacbeats
2) Full details of the weekend are on www.kerouacbeats.blogspot.com PLEASE LOOK
3) The full weekend costs £ 25 but that includes 18 hours of entertainment / free parking / a cuddle with Cristina - and all goes to SMASHSMA
4) Or just come to the Saturday or Sunday at £ 15 per day. I would rather you were there on the Saturday night so you can get a little pisse@@@ drunk and buy things in the SMA auction.
5) It would be amazing for us to meet you but also for you to meet up with people you have been tweeting with for nearly two years now.
6) 6th and 7th JULY - NEW YORK STADIUM / ROTHERHAM
You can buy tickets NOW at
BUY TICKETS NOW - PLEASE
Please come along and say hello - we want to meet you. If you can't make it why not just buy a ticket anyway and consider it a nice mad act to support SMASHSMA
See you there xxx
We always said that it would be terrific if one day we could meet up with some of our strongest ( and maybe strangest ) supporters. Some came to London with us to lobby Nick Clegg. Others have visited us around the country but there are far more of you that I want to say thankyou to in person.
As you are probably aware we recently decided to hold a SMASHSMA fundraising evening. The theme was unusual - we wanted to reproduce a 1950s Beat Generation 'Happening' - complete with bearded poets and musicians. Think MADMEN during the first season and you won't go far wrong.
So after lots of organisation we are holding a full weekend of retro 50s and 60s entertainment at the New York Stadium in Rotherham - South Yorkshire. The theme is based around the Beat Generation writer Jack Kerouac who wrote the famous novel - On The Road.
What's that got to do with SMA ? Nothing - and that's the point. The novel, On the Road, and the philosophy of the Beats was to live life in the moment. to celebrate , to love, to dance and to enjoy every moment - and that's what we think of when we think of Estella. a little girl who made the most out of every ounce of life that was given her - and although that wasn't much in terms of time - it was so much in terms of quality and the impact she has left behind.
So
We want YOU to come and meet Maria and myself and Cristina at the event. We want to thank YOU personally for all you have done and the love and support you have shown. Even if you have never heard of Kerouac then I can still promise you a Saturday experience you will never forget. We have a band Heath Common and the Thin Man who will really get you going, We have a play that I wrote with my mate Brian called Beat Surrender and we have an evening of song, poetry, dance and craziness that will be unlike anything you have ever seen as you are transported back in time to 1957 and a retro night to remember. If you come to both days there are panels and discussions and films about the beats on the Sunday.
Every penny of profit ( please God ) is going to Professor Gillingwater's SMASHSMA research fund.
More importantly though we would love to meet you and thankyou. there will be a film about Estella and the chance to help spread the word about SMA with us. Please come and say hello.
HOW YOU CAN HELP
1) Go and follow the show on TWITTER now @kerouacbeats
2) Full details of the weekend are on www.kerouacbeats.blogspot.com PLEASE LOOK
3) The full weekend costs £ 25 but that includes 18 hours of entertainment / free parking / a cuddle with Cristina - and all goes to SMASHSMA
4) Or just come to the Saturday or Sunday at £ 15 per day. I would rather you were there on the Saturday night so you can get a little pisse@@@ drunk and buy things in the SMA auction.
5) It would be amazing for us to meet you but also for you to meet up with people you have been tweeting with for nearly two years now.
6) 6th and 7th JULY - NEW YORK STADIUM / ROTHERHAM
You can buy tickets NOW at
BUY TICKETS NOW - PLEASE
Please come along and say hello - we want to meet you. If you can't make it why not just buy a ticket anyway and consider it a nice mad act to support SMASHSMA
See you there xxx
Thursday, April 18, 2013
CHANGES
I don't know if you have noticed but we are losing a lot of followers from the Twitter account. Overall the number is increasing but there can be days when we lose over 100 followers as we gain new ones. For a while this upset me then I had a Direct Message from one of our followers who explained that they were leaving now because they 'were not needed any more.'
Then I got it.
There were people thinking that since the birth of Cristina on 20th February we no longer needed help with grieving - we were posting pictures of her and sounding far happier and people were thinking that it was time to go.
They are right in one way. I have certainly been tweeting far less and Maria and I could not be happier with our new life. Cristina is not only healthy, she is beautiful, bright and highly entertaining.
Then I got it.
There were people thinking that since the birth of Cristina on 20th February we no longer needed help with grieving - we were posting pictures of her and sounding far happier and people were thinking that it was time to go.
They are right in one way. I have certainly been tweeting far less and Maria and I could not be happier with our new life. Cristina is not only healthy, she is beautiful, bright and highly entertaining.
Just because we now have Cristina though does not mean we have forgotten about Estella and our promise to SMASH SMA. I have always said that this was not about us it was about raising awareness of this disease and doing all we can to make sure that YOU and other parents don't go through what we did. That is Estella's legacy.
Things have gone quiet but we are just regrouping thoughts and ideas - at the moment
We are organising a literature convention on July 6 and 7 at the New York Stadium in Rotherham. Its based around the works of American writer and poet Jack Kerouac and all proceeds will go to Tom Gillingwater's SMA research. Even if you have never heard of Kerouac there's a concert, a film, a play , a party and a great opportunity to meet Maria and me - and to help towards SMA research. There's more details at www.kerouacbeats.blogspot.co.uk or follow the twitter account @kerouacbeats
We are still awaiting more news on the political side. Norman Lamb and Nick Clegg are pushing the screening consultation and new MP Sarah Champion is also promoting our cause.
The Jennifer Trust have announced that they are now supporting Tom Gillingwater
We are getting great celebrity support with daily messages from Lauren Carre who has been a great follower
Wrist bands are still selling to raise funds.
To be very clear - we still need your support because the campaign is now about making sure that this disease is seeing its last days. So if we look happy - we are - but that doesn't mean that every single day there are not new babies diagnosed with SMA - and that's what we want to stop.
Cristina had her 8 week check up on Monday.
Last time we had an 8 weeks check up was the day they diagnosed Estella and our world ended.
She passed with flying colours.
Please stay - we need you
Saturday, February 2, 2013
NOT A LOSER
Yesterday I got one of those heartbreaking messages that I have seen all too often during the last year.A Dad wrote to tell me that he had lost his son aged 18 months to SMA. I wrote back with my condolences and my usual offer of any help and support and then received a response asking how to cope with the loss.
I will not go into this individual case but lay awake last night I did wonder if it was worth putting a few notes on here that may one day help somebody with their loss.
The first thing to say is that I am very aware that every circumstance is different and I know there is lots of amazing professional help out there. I would not dream of trying to say that there is a particular way of dealing with the death of a child. There simply isn't.
What I can do is give you some thoughts as to what happened with Estella and hope that some of these words can make a small difference.
I know the reason we get asked how to handle this particular situation is because it appears that we have managed to handle the events that occurred in a particular way. I will elaborate on that later.
Here's how I see things.
There is nothing more devastating than losing your child. It is more than a cliche to say that it is unnatural for a parent to outlive their child but cliches exist for a reason and the first feeling when you lose a child is the sense of unfair and harrowing loss. I can not even begin to imagine how it must feel to lose a child suddenly - as in the case of an accident - no time to prepare. No time to even wonder about any consequence.
We lost Estella over a period of six months from her diagnosis at 8 weeks to her death at 8 months. That period of time allows for a lot of talking and a lot of planning but you are still not prepared or ready as the day your little girl says goodbye is not known. SMA is a cruel and heartless disease. The best way I can describe what happens is that there are a number of very bad days when Estella had some occurrence such as a blockage that prevented her breathing that she overcame. Upon overcoming though she never returned to the same health she had before the attack. You don't see a gradual deterioration you have days when certain skills and abilities are lost never to be recovered. The blessing is that this gives you the pleasure of many good moments and many triumphs and some of my happiest memories are of singing to the little tinker or just sitting for hours stroking her hand. In every moment of pleasure though there is an enormous black hole inside your soul that aches beyond anything I can ever explain. It is constant. It is frightening and it is tragic. You are watching your daughter saying goodbye at the same time as you are watching her grow and develop. There are many moments of silence when you sit alone and scream inside.
Eventually though we had to say goodbye to Estella. There is a longer description elsewhere in this blog of that Sunday afternoon for the purpose of this let me just remind you that her death was painless , measured, timely and beautiful. We were able to hold her one last time without her breathing mask and the machines and the tubes and for one last time we were able to kiss her and say our final goodbyes. The word beautiful sometimes confuses people and may even offend them. Let me explain. We had had many meetings with people who explained all of the possible options and scenarios that could happen at the end and believe me there were some horrific ones. In the end we had the best possible farewell. Maria held Estella. I held Maria and we saw our daughter fall asleep. We felt her breath and watched her calmly pass with no pain and none of the horrific possibilities that we had been prepared for, We bathed her. We held her. We dressed her. We said farewell.
So what happens next.I still say that one of the most terrible moments I experienced throughout the whole six months was the unbelievable discussions where you have to sit in a room preparing all of the details for your daughter's funeral. The decisions. The choices. The numb disbelief that you really are talking to people about the type of coffin and the music and the service. Beyond the funeral though there comes the worst bit - the loss.
OK. You leave the crematorium without your little girl. You have to walk away leaving her behind. You're not going to see her ever again. It is the emptiest and most barren feeling anybody could ever experience. You drive away without your little girl.
There is then no pathway that anybody can ever tell you how to tread.
I felt guilty. I am a big hunking lump and I could not protect my little girl from this ending. Nothing I could do could make this disease go away. I promised her I would do what i could to try and help others but there was nothing I could do. I could not help Maria with her loss other than realising whatever I was feeling this generous, gentle perfect Mummy was feeling a hundred times more. I was useless at caring for Estella. Some days I would have to go and walk around the gardens of Bluebell Wood for an hour but while I did that Maria looked after every tube , mask, food issue - Maria very kindly always said that she was maintenance and I was entertainment.I got a couple of hours a day to read Cat in the Hat to Estella and I watched her eyebrow raise in speculative laughter at my tales and songs and tickles. Part of my guilt was wondering if I had done enough as a Daddy. At worst I would think to myself that what I gave to Estella was a disease that was in my genes. That was my present. When those kind of thoughts crept in I managed to deal with them by a kind of logic. Maria also carried SMA so if it was my fault was it also hers ? Of course not that would be stupid to think - so if that was the case then it wasn't my fault either.
You feel guilty though. You feel guilty any time life becomes normal. I remember watching an episode of Father Ted and laughing at some silly Mrs Doyle comment and then absolutely hating myself for daring to laugh when my daughter was dead. There is one way of thinking though that Maria taught me ( are you starting to grasp how strong she is ) - all she said to me was , "Is this how Estella would want it ?" It's a perfect way of thinking. Would that clever and intelligent and sensitive little girl want Maria and I to be unhappy forever or would she want life to carry on. Would she want us to remember her with fear and whispers or with happiness and wonder?
The next fact is that if you lose a child you are about to go through the biggest challenge to your marriage that can possibly exist. Many couples end up divorced. Fact. I think we survived for a very simple reason - no matter how low we got we never - never-never blamed each other for anything that happened. I blamed myself. Maria had days when she blamed herself but there was never an instant when I blamed Maria and she never ever blamed me. Maria gave Estella the greatest, most amazing support possible. She lived by Estella's side, She kept me and her parents sane. She made decisions. She fought for Estella when she needed to fight. She kept her alive at least a dozen times. Never blame each other.
SMASHSMA helped. It gave a focus and passed many hours building up the awareness that is explained in other sections. The point here that I would suggest is that there will be people who want to help you. Let them because they are exceptional people. Some people will not know how to approach you or what to say. Of course they don't - its an experience that they will hopefully never have to feel. Don't turn them away though - take all the support you can. It makes you stronger.
There will be certain days. Anniversaries. Birthdays. Christmases - so many occasions and moments. Handle them by accepting you will feel bad. Lock yourself away. Take yourselves away. You know those days will hurt so don't pretend that they will not.
The one thing I don't know how to prepare for though is the thing that happened a couple of hours ago. That thing will happen thousands of times.
I was driving along the motorway. A winter afternoon. Listening to football on the radio. Looking at the traffic. Mind in neutral. Then I noticed how gorgeous the sun seemed streaming through the trees to my left as I drove. Winter sun is white and sudden and hauntingly beautiful.
Bam - thirty seconds later I was still driving with face soaked in tears and my heart pounding. Total loss of sense and calm as from somewhere buried within I could see , hear , touch , smell and taste every single thing about Estella. It came from nowhere. No preparation. Crying Muppet Daddy filled with memory and sensitivity.Somewhere within the neurons connect and all of the pain and all of the hope and all of the memory and all of the tragic silent certainty of death comes howling into your heart and soul. I can't prepare anybody for that and do you know - I wouldn't want to. I would not want those moments to stop ever because when they happen I can.. feel her again. The tears flow and the emotions rage because in the end all of the techniques and all of the coping strategies and all of the logic is absolute nonsense. You lost the most treasured love you will ever know and you are not supposed to accept it - you are not supposed to block it - you are not supposed to forget it.
How to cope with those moments. Be grateful that you can feel. be grateful that you can remember. Be grateful that for 30 seconds she is with you again. That's the way I flip it to welcome the sadness because always, always, always when the tears stop , when the shaking stops, when the heart beats still again you are left with the warmest of feelings. You are left with the glowing memory of the reality of the child you lost
And every time that happens you find her again. I'm a soppy git - I see these moments as Estella coming to visit me and when she does ? She doesn't want to see her Daddy crying - she doesn't want to see him upset so if you ever see a wet faced Muppet singing Puff the Magic Dragon or Moon River driving down the motorway don't point and laugh too loud - it's just me or somebody like me remembering better days .
I don't know if any of that helps. I hope so. What I will say is what I always say. I will do what I can to rid the world of SMA but in the meantime if there are any parents that I can help in any way - then the answer is very simple. tell me where - tell me when and I'll be there.
Maria and I decided very early on not to go along to support groups and memorial groups and the like - it's not us. We visit Estella. We talk to her. We're even making another baby :) for her to laugh at and look after but as for dealing with losing Estella - we will not be able to do that ever. Part of being a Muppet Daddy and a Cuddly Mummy is refusing to accept that we have lost her. I don't particularly have religious views - sorry I don't - but what I do know is that life goes on, nature is beautiful and by some unbelievable wonderful quantum physics riddle of the universe kind of thingy - ( technical term ) I will hold her again . I will.
I miss her but she came to visit two hours ago. Tricky little Tinker.
I will not go into this individual case but lay awake last night I did wonder if it was worth putting a few notes on here that may one day help somebody with their loss.
The first thing to say is that I am very aware that every circumstance is different and I know there is lots of amazing professional help out there. I would not dream of trying to say that there is a particular way of dealing with the death of a child. There simply isn't.
What I can do is give you some thoughts as to what happened with Estella and hope that some of these words can make a small difference.
I know the reason we get asked how to handle this particular situation is because it appears that we have managed to handle the events that occurred in a particular way. I will elaborate on that later.
Here's how I see things.
There is nothing more devastating than losing your child. It is more than a cliche to say that it is unnatural for a parent to outlive their child but cliches exist for a reason and the first feeling when you lose a child is the sense of unfair and harrowing loss. I can not even begin to imagine how it must feel to lose a child suddenly - as in the case of an accident - no time to prepare. No time to even wonder about any consequence.
We lost Estella over a period of six months from her diagnosis at 8 weeks to her death at 8 months. That period of time allows for a lot of talking and a lot of planning but you are still not prepared or ready as the day your little girl says goodbye is not known. SMA is a cruel and heartless disease. The best way I can describe what happens is that there are a number of very bad days when Estella had some occurrence such as a blockage that prevented her breathing that she overcame. Upon overcoming though she never returned to the same health she had before the attack. You don't see a gradual deterioration you have days when certain skills and abilities are lost never to be recovered. The blessing is that this gives you the pleasure of many good moments and many triumphs and some of my happiest memories are of singing to the little tinker or just sitting for hours stroking her hand. In every moment of pleasure though there is an enormous black hole inside your soul that aches beyond anything I can ever explain. It is constant. It is frightening and it is tragic. You are watching your daughter saying goodbye at the same time as you are watching her grow and develop. There are many moments of silence when you sit alone and scream inside.
Eventually though we had to say goodbye to Estella. There is a longer description elsewhere in this blog of that Sunday afternoon for the purpose of this let me just remind you that her death was painless , measured, timely and beautiful. We were able to hold her one last time without her breathing mask and the machines and the tubes and for one last time we were able to kiss her and say our final goodbyes. The word beautiful sometimes confuses people and may even offend them. Let me explain. We had had many meetings with people who explained all of the possible options and scenarios that could happen at the end and believe me there were some horrific ones. In the end we had the best possible farewell. Maria held Estella. I held Maria and we saw our daughter fall asleep. We felt her breath and watched her calmly pass with no pain and none of the horrific possibilities that we had been prepared for, We bathed her. We held her. We dressed her. We said farewell.
So what happens next.I still say that one of the most terrible moments I experienced throughout the whole six months was the unbelievable discussions where you have to sit in a room preparing all of the details for your daughter's funeral. The decisions. The choices. The numb disbelief that you really are talking to people about the type of coffin and the music and the service. Beyond the funeral though there comes the worst bit - the loss.
OK. You leave the crematorium without your little girl. You have to walk away leaving her behind. You're not going to see her ever again. It is the emptiest and most barren feeling anybody could ever experience. You drive away without your little girl.
There is then no pathway that anybody can ever tell you how to tread.
I felt guilty. I am a big hunking lump and I could not protect my little girl from this ending. Nothing I could do could make this disease go away. I promised her I would do what i could to try and help others but there was nothing I could do. I could not help Maria with her loss other than realising whatever I was feeling this generous, gentle perfect Mummy was feeling a hundred times more. I was useless at caring for Estella. Some days I would have to go and walk around the gardens of Bluebell Wood for an hour but while I did that Maria looked after every tube , mask, food issue - Maria very kindly always said that she was maintenance and I was entertainment.I got a couple of hours a day to read Cat in the Hat to Estella and I watched her eyebrow raise in speculative laughter at my tales and songs and tickles. Part of my guilt was wondering if I had done enough as a Daddy. At worst I would think to myself that what I gave to Estella was a disease that was in my genes. That was my present. When those kind of thoughts crept in I managed to deal with them by a kind of logic. Maria also carried SMA so if it was my fault was it also hers ? Of course not that would be stupid to think - so if that was the case then it wasn't my fault either.
You feel guilty though. You feel guilty any time life becomes normal. I remember watching an episode of Father Ted and laughing at some silly Mrs Doyle comment and then absolutely hating myself for daring to laugh when my daughter was dead. There is one way of thinking though that Maria taught me ( are you starting to grasp how strong she is ) - all she said to me was , "Is this how Estella would want it ?" It's a perfect way of thinking. Would that clever and intelligent and sensitive little girl want Maria and I to be unhappy forever or would she want life to carry on. Would she want us to remember her with fear and whispers or with happiness and wonder?
The next fact is that if you lose a child you are about to go through the biggest challenge to your marriage that can possibly exist. Many couples end up divorced. Fact. I think we survived for a very simple reason - no matter how low we got we never - never-never blamed each other for anything that happened. I blamed myself. Maria had days when she blamed herself but there was never an instant when I blamed Maria and she never ever blamed me. Maria gave Estella the greatest, most amazing support possible. She lived by Estella's side, She kept me and her parents sane. She made decisions. She fought for Estella when she needed to fight. She kept her alive at least a dozen times. Never blame each other.
SMASHSMA helped. It gave a focus and passed many hours building up the awareness that is explained in other sections. The point here that I would suggest is that there will be people who want to help you. Let them because they are exceptional people. Some people will not know how to approach you or what to say. Of course they don't - its an experience that they will hopefully never have to feel. Don't turn them away though - take all the support you can. It makes you stronger.
There will be certain days. Anniversaries. Birthdays. Christmases - so many occasions and moments. Handle them by accepting you will feel bad. Lock yourself away. Take yourselves away. You know those days will hurt so don't pretend that they will not.
The one thing I don't know how to prepare for though is the thing that happened a couple of hours ago. That thing will happen thousands of times.
I was driving along the motorway. A winter afternoon. Listening to football on the radio. Looking at the traffic. Mind in neutral. Then I noticed how gorgeous the sun seemed streaming through the trees to my left as I drove. Winter sun is white and sudden and hauntingly beautiful.
Bam - thirty seconds later I was still driving with face soaked in tears and my heart pounding. Total loss of sense and calm as from somewhere buried within I could see , hear , touch , smell and taste every single thing about Estella. It came from nowhere. No preparation. Crying Muppet Daddy filled with memory and sensitivity.Somewhere within the neurons connect and all of the pain and all of the hope and all of the memory and all of the tragic silent certainty of death comes howling into your heart and soul. I can't prepare anybody for that and do you know - I wouldn't want to. I would not want those moments to stop ever because when they happen I can.. feel her again. The tears flow and the emotions rage because in the end all of the techniques and all of the coping strategies and all of the logic is absolute nonsense. You lost the most treasured love you will ever know and you are not supposed to accept it - you are not supposed to block it - you are not supposed to forget it.
How to cope with those moments. Be grateful that you can feel. be grateful that you can remember. Be grateful that for 30 seconds she is with you again. That's the way I flip it to welcome the sadness because always, always, always when the tears stop , when the shaking stops, when the heart beats still again you are left with the warmest of feelings. You are left with the glowing memory of the reality of the child you lost
And every time that happens you find her again. I'm a soppy git - I see these moments as Estella coming to visit me and when she does ? She doesn't want to see her Daddy crying - she doesn't want to see him upset so if you ever see a wet faced Muppet singing Puff the Magic Dragon or Moon River driving down the motorway don't point and laugh too loud - it's just me or somebody like me remembering better days .
I don't know if any of that helps. I hope so. What I will say is what I always say. I will do what I can to rid the world of SMA but in the meantime if there are any parents that I can help in any way - then the answer is very simple. tell me where - tell me when and I'll be there.
Maria and I decided very early on not to go along to support groups and memorial groups and the like - it's not us. We visit Estella. We talk to her. We're even making another baby :) for her to laugh at and look after but as for dealing with losing Estella - we will not be able to do that ever. Part of being a Muppet Daddy and a Cuddly Mummy is refusing to accept that we have lost her. I don't particularly have religious views - sorry I don't - but what I do know is that life goes on, nature is beautiful and by some unbelievable wonderful quantum physics riddle of the universe kind of thingy - ( technical term ) I will hold her again . I will.
I miss her but she came to visit two hours ago. Tricky little Tinker.
Subscribe to:
Posts (Atom)